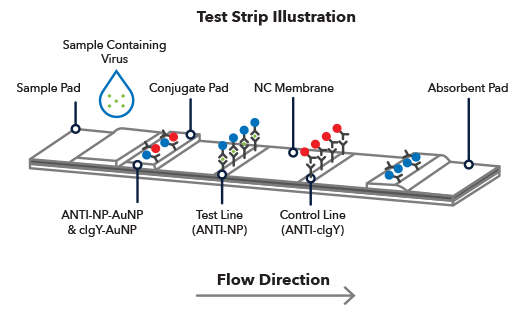
lateral flow assay development
Qualification of Critical Reagents for Lateral Flow Assay Development. In this study a colloidal gold nanoparticle-based lateral-flow AuNP-LF assay was developed to achieve rapid diagnosis and on-site detection of the IgM antibody against the.
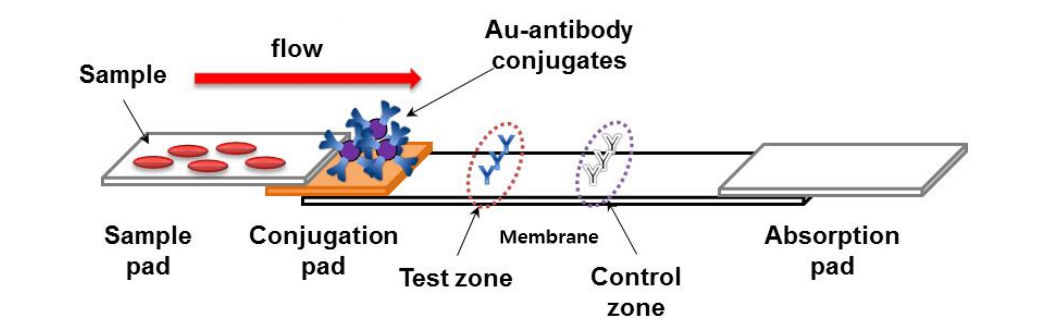
Lateral Flow Immunoassay Creative Diagnostics
When 100 of a food containing an allergen is tested the.
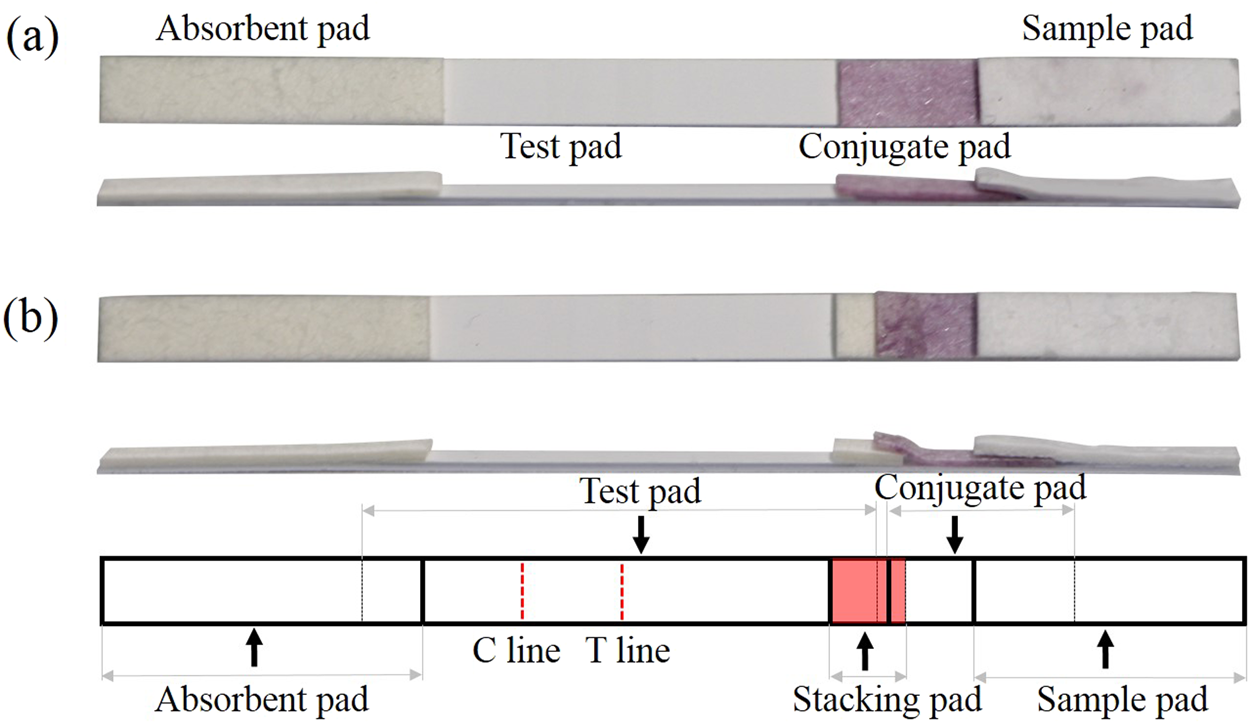
. Introduction to Lateral Flow OVERVIEW Figure 1 Figure 1. Workflows to guide the LFA development process exist but moving from target selection to an LFA that is ready for field testing can be labor intensive resource heavy and time consuming. Gold colloid silver platinum latex colored fluorescent and magnetic Development of multiple assay formats.
DCN Dx develops lateral flow flow-through dry chemistry and other assays for rapid diagnostic tests around a variety of conjugate labels and reporting requirements. A successful diagnostic lateral flow assay is the product of many small optimizations that vary depending on the particle type target and system. Lateral Flow Development and Manufacturing Journey Quality Partnership Results Innovation Shaun Phillips RD Senior Scientist Contract RD overview.
The qualification of antibodies and antigensoften called critical reagentsis complicated and. Jersey City NJ Ellume. Using the latest RDS-2500 features and lateral flow processing algorithms this universal lateral flow assay development kit is perfect for start-ups.
Lateral flow assays LFAs are rapid and inexpensive diagnostic devices that can be used to test for a target substance analyte in a sample. The existing standard method of detection for this food safety assay requires multiple days and a skilled scientist to perform the test. Lateral Flow A technique known as the hook effect can produce false negative results if the allergen is overloaded in the device.
Lateral Flow Assay Development Kit. Lateral Flow Assay Development Guide. The development of Lateral Flow Immunochromatography Assay can be divided into two levels.
Lateral flow assay LFA has made a paradigm shift in the in vitro diagnosis field due to its rapid turnaround time ease of operation and exceptional affordability. Standardizing membrane characteristics and optimizing molecular level immunoassay reaction. Using ultra-sensitive nanoparticle technologies.
A commercial pregnancy test which uses 40 nm gold nanoparticles as the detection label. The development of Lateral Flow Immunochromatography Assay can be divided into two levels. In-house high quality reagent and conjugate preparation.
Standardizing membrane characteristics and optimizing molecular level. Lateral flow assay development is the successful production of a simple to use diagnostic test for validating the presence or absence of a wide range of pathogens. Combined with recent entrants to the lateral flow development and manufacturing ecosystem such as Luminostics Inc.
Lateral Flow Development Start to Finish Lateral flow rapid test development at Abingdon Health typically follows a number of stages with formal reviews at critical points. The faint red line indicates that the. Some of the advantages to the LFA.
Global Lateral Flow Assay Testing Market Research 2022-2029 provides exhaustive analysis of business statistics trends recent developments and future scope of industry. Milpitas CA Celltrion USA Inc. Bio-Techne is a leading provider of bio-reagents and analytical instrumentation and now provides lateral flow assay development services including.
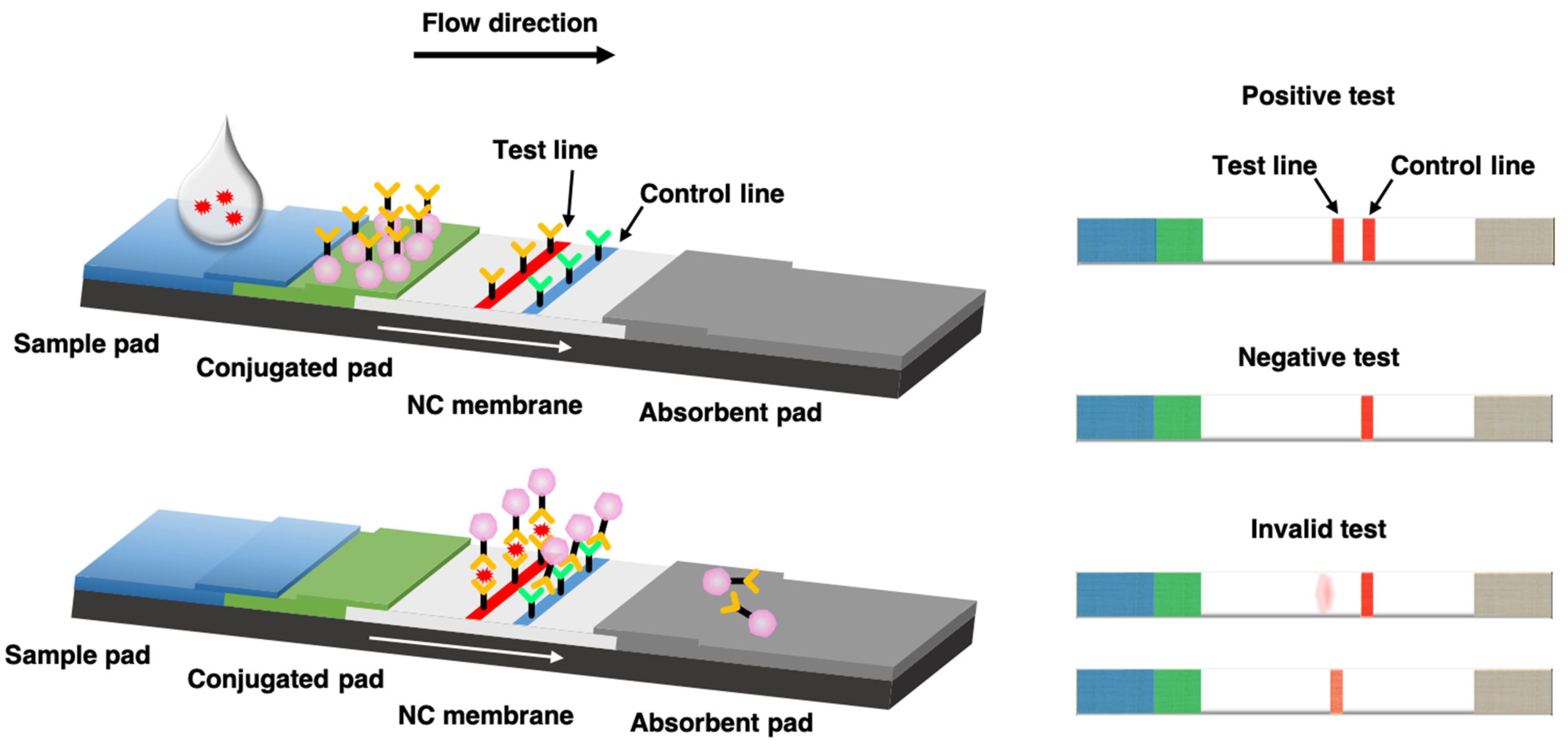
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html

Development Of A Lateral Flow Strip Membrane Assay For Rapid And Sensitive Detection Of The Sars Cov 2 Analytical Chemistry
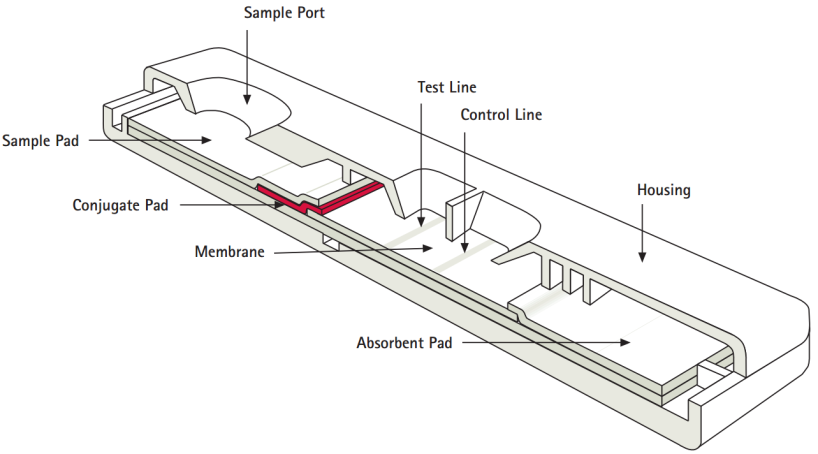
Test Assay Development Technical Platform Cd Bioparticles

Capillary Flow Control In Lateral Flow Assays Via Delaminating Timers Science Advances

Lateral Flow Assays And Applications Joysbio Biotechnology
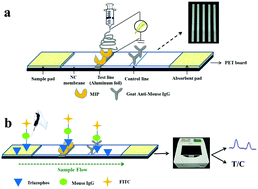
Development Of Fluorescent Lateral Flow Test Strips Based On An Electrospun Molecularly Imprinted Membrane For Detection Of Triazophos Residues In Tap Water New Journal Of Chemistry Rsc Publishing
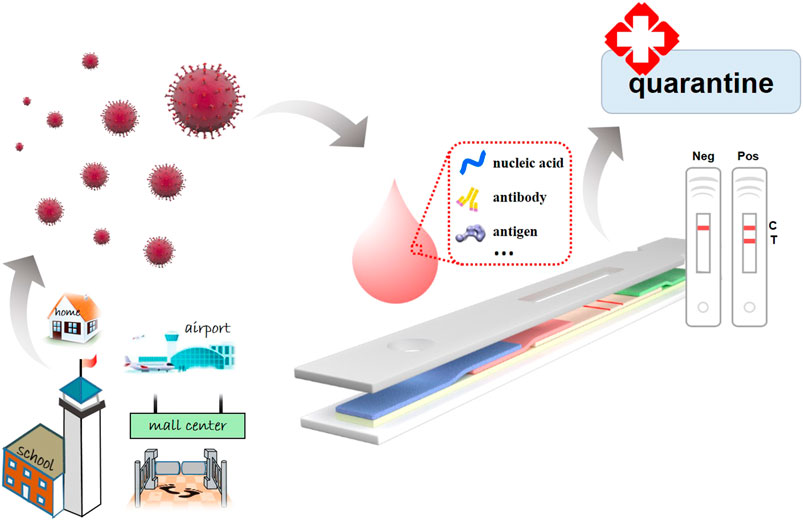
Frontiers Recent Progress On Rapid Lateral Flow Assay Based Early Diagnosis Of Covid 19

Lateral Flow Assay Products Cytodiagnostics Inc

How To Develop Rapid Test Based On Immunochromatography Ballya

Universal Lateral Flow Assay Kit Cytodiagnostics Inc

Materials And Equipment Of Lateral Flow Assay Development Antiteck
A Lateral Flow Assay For Quantitative Detection Of Amplified Hiv 1 Rna Plos One
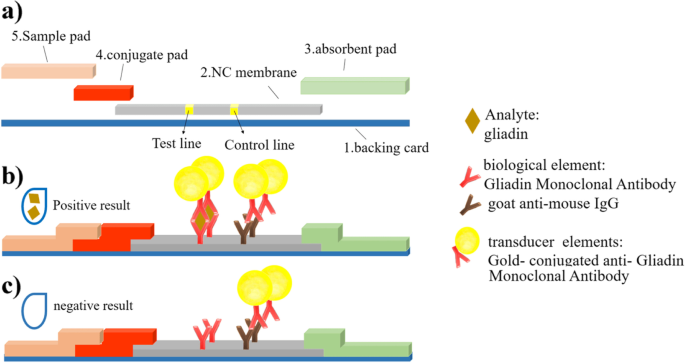
Gold Based Nanoplatform For A Rapid Lateral Flow Immunochromatographic Test Assay For Gluten Detection Bmc Biomedical Engineering Full Text
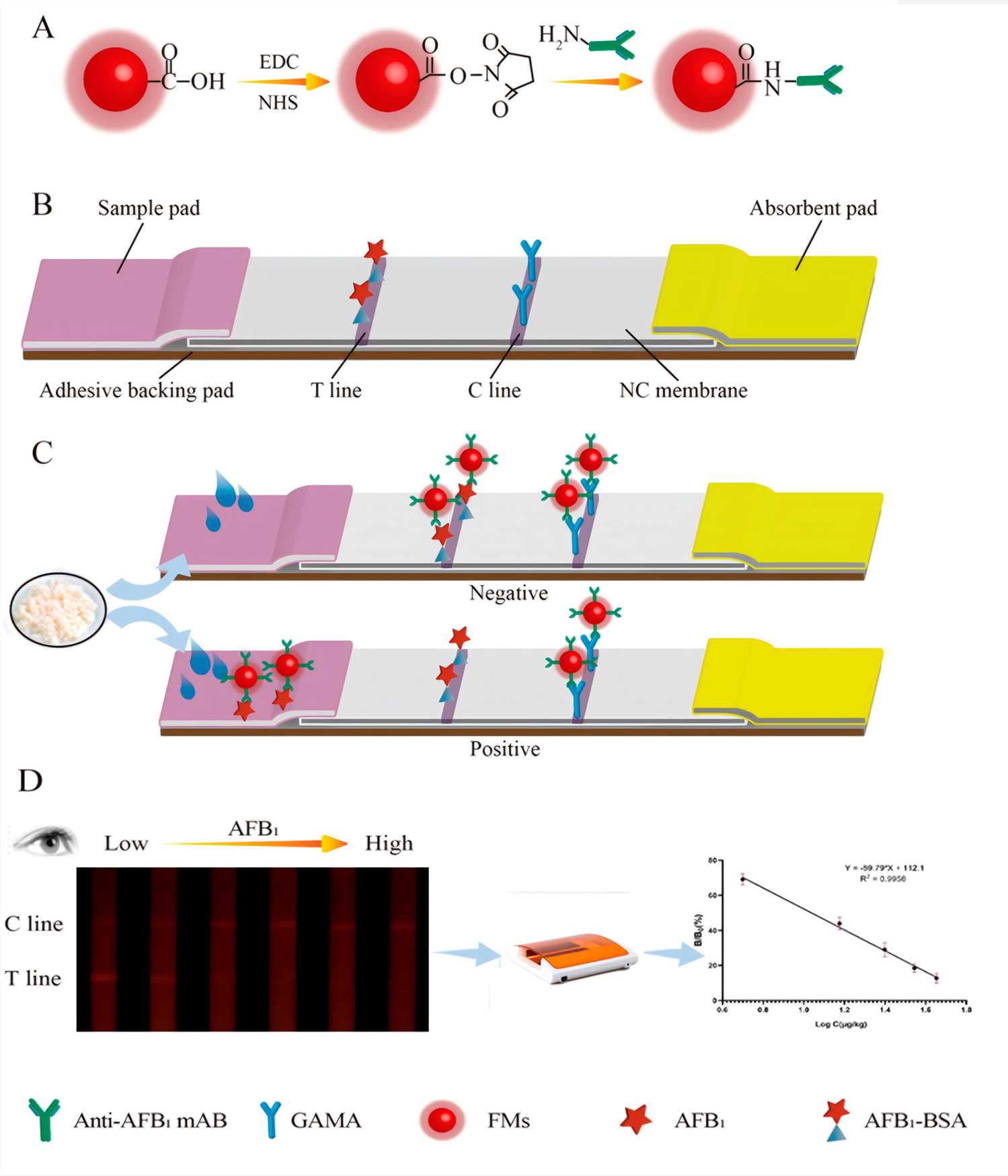
Lateral Flow Immunochromatographic Assay Lfia Based Kits Development Creative Biolabs
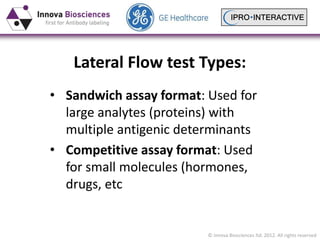
Lateral Flow Assay Development And The Use Of Gold Nanoparticles

Lateral Flow Reagent Dispenser Alfrd Claremontbio Com

Ultrasensitive And Highly Specific Lateral Flow Assays For Point Of Care Diagnosis Acs Nano

Lateral Flow Immunoassay Integrated With Competitive And Sandwich Models For The Detection Of Aflatoxin M1 And Escherichia Coli O157 H7 In Milk Journal Of Dairy Science